Abstract
Introduction: Prognosis and response to Multiple Myeloma (MM) therapy can vary markedly even among patients at the same disease stage and with comparable clinical characteristics. This heterogeneity is driven by tumor intrinsic features such as somatic alterations impacting the genetic and epigenetic landscape of a patient as well immune dysfunction in the tumor microenvironment (TME). Tumor gene expression profiles have been correlated with clinical outcomes and are potentially useful for precision medicine approaches in MM. In this study, we hypothesized that clinical outcomes and variable responses to therapeutic modalities in MM may be driven by expression of critical gene sets, and sought to identify clinically actionable molecular subtypes using the available RNAseq data from newly diagnosed MM (NDMM) patients in the COMPASS functional genomics study.
Methods: Unsupervised machine learning approach based on consensus non-negative matrix factorization (cNMF) was applied to the most variable genes (n=6019; top 10%) based on median absolute deviation (MAD) of log2(TPM) in the RNAseq data derived from CD138 positive bone marrow patient samples collected at baseline (N=766) to identify transcriptomic-based molecular subtypes. After the optimal number of clusters was determined using their cophenetic coefficient, the subtypes were assessed for their association with clinical outcomes in patients with available data (N=753). Survival analyses included Kaplan-Meier (KM) curves, log-rank tests, univariate and multivariate Cox regression, and Wald-tests. Subtypes were also biologically characterized by gene signature scoring using well-curated pathway signatures (GSVA analysis using Hallmark pathways), cell type enrichment (xCell enrichment).
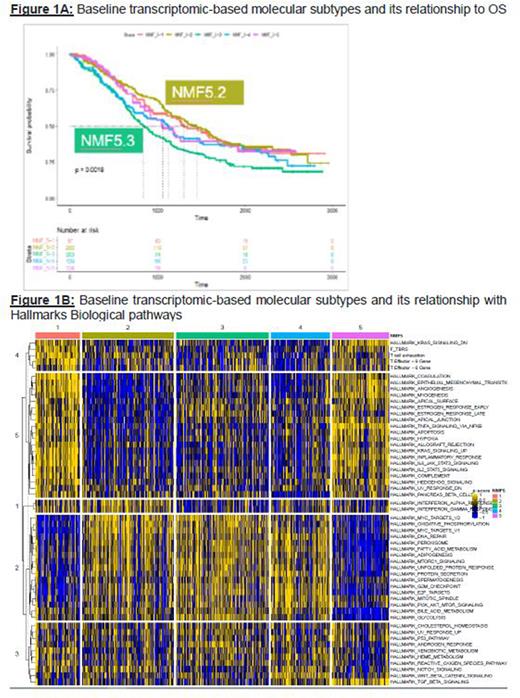
Results: Our cNMF based analysis identified five clusters of patients based on the 6,019 (top 10%) most variable genes. These novel subtypes (NMF5.1-5) had unique biology associated with time-to-second-line of therapy (Figure 1A, log rank p=0.0018). Biological differences between NMF5.3 and NMF5.2 were investigated further to understand mode of action and mechanism of resistance to IMiD/PI treatment in NDMM patients.
NMF5.2 was the best prognostic cluster and associated with the longest time to 2nd line (median time to 2nd line 1,444 days) while NMF5.3 is associated with the shortest time to 2nd line (831 days). NMF5.2 was characterized with a more intact immune system including higher immune cell activation and immune microenvironment scores as well as higher levels in the INF pathway (Hallmark Interferon gamma and alpha pathways Wilcoxon p-value<0.001). NMF5.3, on the other hand, displayed lower scores for immune activation and had more of immunosuppressive/ exhausted immune microenvironment (Wilcoxon p-value<0.001). Ongoing analyses to evaluate the baseline expression level of immunotherapeutic targets in MM like BCMA, FcRH5, GPRC5D and additional immune checkpoint antigens and its association with NMF5.2 and 3 will also be discussed.
Conclusions: Unique molecular subtypes identified via a novel unsupervised machine learning may have implications on our understanding of how NDMM patients respond to existing therapies and suggest that the addition of immune checkpoints can improve patients’ clinical outcome.
Disclosures
Hamidi:Genentech: Current Employment, Current equity holder in publicly-traded company. Sumiyoshi:Roche/Genentech: Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months; Genentech: Current Employment, Ended employment in the past 24 months, Research Funding. Dos Santos:Genentech: Current Employment, Current equity holder in publicly-traded company. Tracy:Genentech, Inc.: Current Employment; F. Hoffmann-La Roche: Current equity holder in publicly-traded company. Cho:Takeda: Other: Receive laboratory research support from the above companies. Salary value is less than $10,000 per company., Research Funding; BMS/Celgene: Other: Receive laboratory research support from the above companies. Salary value is less than $10,000 per company., Research Funding. Hilz:Genentech: Current Employment, Current equity holder in publicly-traded company. Chopra:Roche, BMS, Amgen: Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months, Patents & Royalties: Publication number: 20210100805 Publication number: 20200405737 Patent number: 10695352 Patent number: 10653710 Publication number: 20180303840 Publication number: 20180296583; Genentech Inc. (Roche): Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal